Ensuring bioavailability and solubility of your NCEs with spay drying technology
The pharmaceutical industry is witnessing a huge demand for Spray Drying services in recent times. A major reason for this is the rising trend of developing new chemical entities (NCEs), which invariably have bioavailability and solubility issues. Pharmaceutical spray drying technology which can enhance NCEs’ bioavailability and solubility, has found wide acceptance in the scientific community compared to other technologies.
However, while many CDMOs have added spray drying capabilities to their service portfolio, few can handle flammable solvents in the development lab. Yet another issue is the ability to scale up volumes to meet early-phase clinical study requirements.
At Syngene, we have a dedicated GMP facility to handle spray drying equipment and process for NCEs containing flammable solvents to cater to the requirements of early-phase clinical study. Our highly trained team has the expertise to screen the ‘right’ solvents, select well-suited polymers, ensure the ‘right’ polymer-to-drug ratio, adjust appropriate solid content, and follow all process parameters. Only then can you get a molecule that has the right targeted product characteristics (amorphous form) to meet patient requirements. Further, our drug substance, drug product, and solid-state characterization teams work in an integrated manner to create high-quality drugs, including converting them to a viable dosage form that is efficient and effective.
In summary, a good balance of safety, quality compliance, and technological advancement make Syngene the preferred CDMO for early-phase studies.
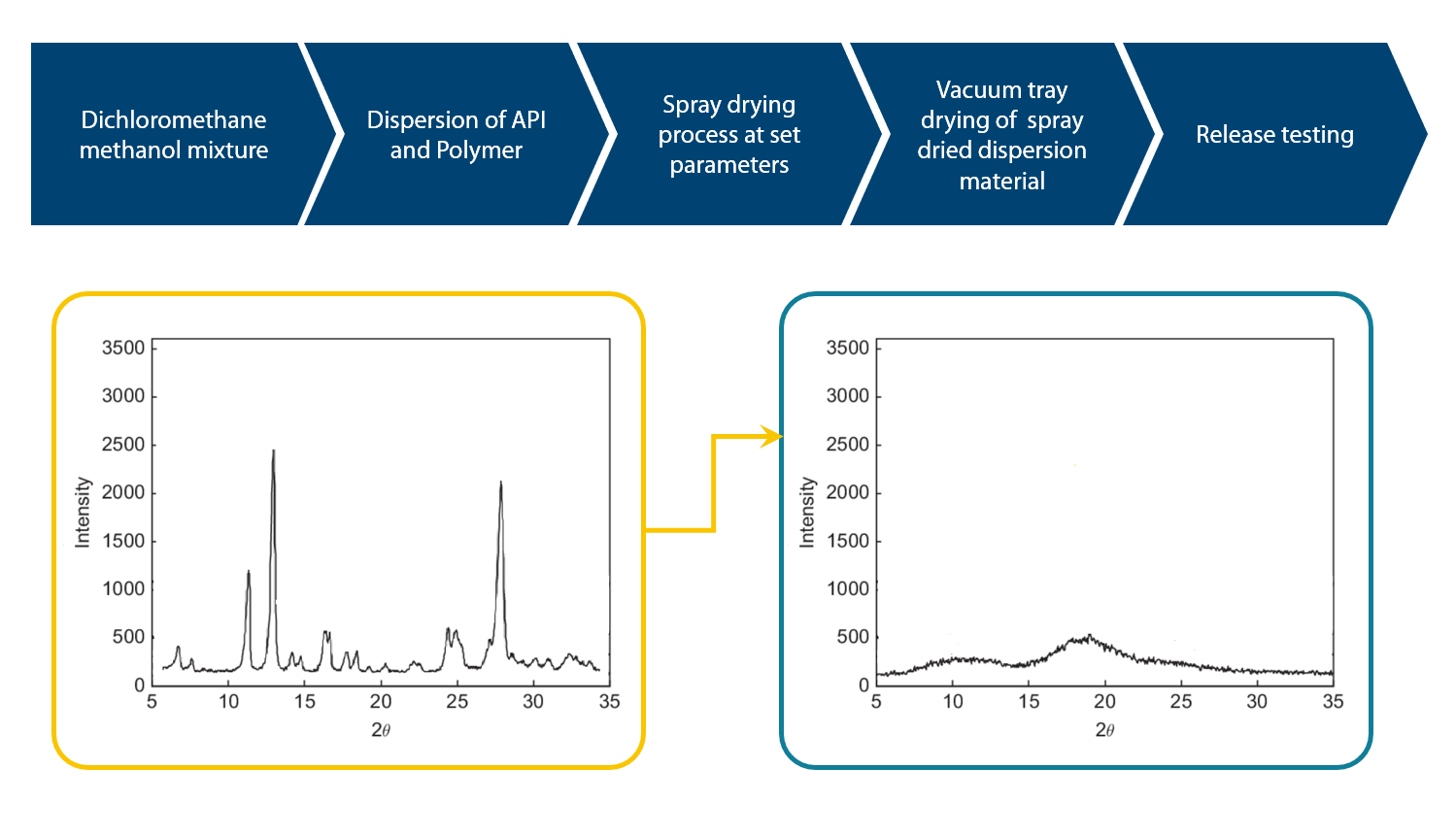
Figure 1: X-ray diffraction pattern of API: Pre and post spray drying



