Introduction
Design of an Experiment (DoE) is achieving increasing acceptance in the pharmaceutical sector1 because it is being seen as an efficient tool for process optimization. DoE is the main component of a statistical toolbox that can make controlled changes in input variables to gain maximum information on cause and effect relationships while using minimal resources.1.
Advantages of DoE
DoE has several advantages when it comes to development of quality-oriented products. These are as follows:
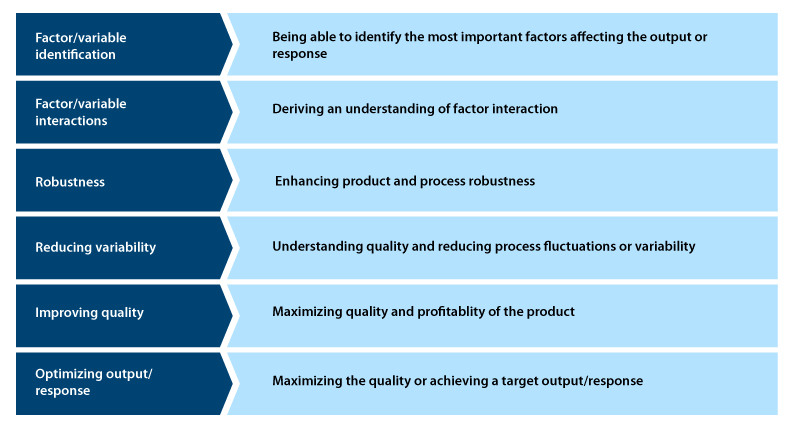
Significance of DoE in process development
- Helps to establish cause and effect relationships through mathematical models
- Helps to identify uncontrollable or unknown parameters
- Provides accurate information to design and develop new processes and products, thus minimizing the time and resource requirements.
- Ensures good performance for an existing process
- Achieves product and process robustness
Steps in DoE
DoE comprises nine distinct steps. These are as follows:
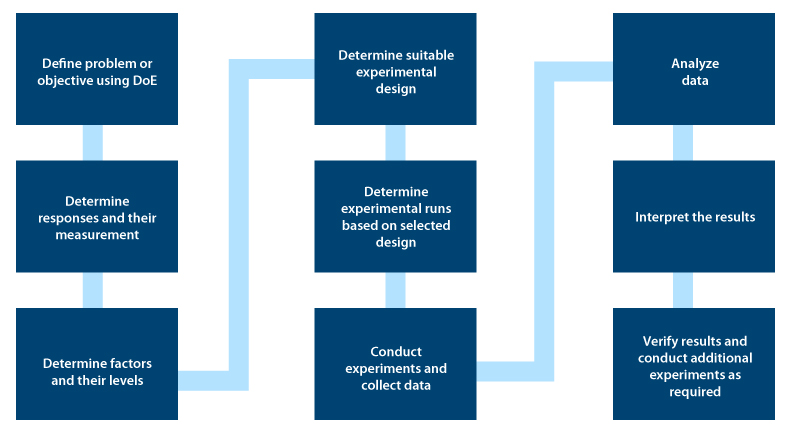
Executing DoE
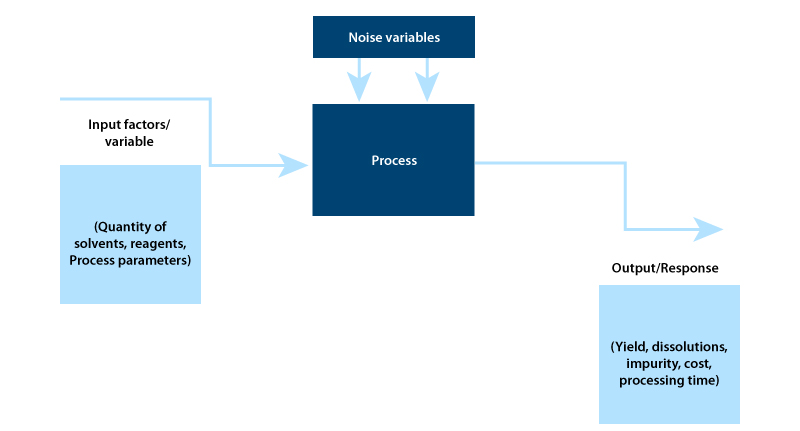
Setting an objective: This is the first step in DOE. You need to clearly define Quality Target Product Profile (QTPP) using information and knowledge from scientific literature and technical experiences.
Identifying the process parameters and responses based on the DoE objective: During this stage, DoE identifies the cause and effect relationship between process parameters and responses.
Developing experimental design: In this stage, you need to identify the factors that influence responses and categorize controlled and uncontrolled variables known as screening. You should further optimize the process based on the Pareto principle that 20 % of the factors are responsible for 80 % of the responses. The impact of controlled factors on product quality can be identified in this stage.
Executing the Design: Based on the DoE objective, the generated design should be accurately executed, while making sure uncontrolled factors are identified and kept constant.
Checking for consistency in data sets: Based on the experimental assumption, you need to check for consistency in data sets. The generated design should be accurately executed.
Analyzing results: You need to use statistical models like ANOVA (Analysis of Variance) to help identify the effect of significant factors and their interactions in eliciting a response.
Interpreting results: At this stage, final responses are evaluated to decide on the following activities – Executing confirmed experimental runs, scale-up, and technology transfer activities.
Types of DoE:
- Factorial experiments
- Full factorial experiments
- Here, all treatment combinations that are associated with factors and levels are studied. This includes the evaluation of individual effects of each factor and their interactions
- When the number of levels or levels of factors or both, increase, the number of treatment combinations that need to be included in the experiments also increase. The statistical analysis then becomes complex and results in wastage of time and experimental material
- Fractional factorial experiments
- This mainly focuses on selecting an experimental design that evaluates the most significant effects and interactions
- It cannot evaluate all effects and interactions
- Full factorial experiments
- Screening Experiments: This comprises screening the factors or variables in the process and determining critical variables that affect process response.
- Plackett-Burman Designs: Developed by Plackett and Burman in the 1940s, this design approach is efficient because it assumes all interactions are negligible compared to the main effects
- Taguchi’s Orthogonal Arrays: Dr.Taguchi modified the Plackett-Burman design approach so that an experimenter could assume that interactions are not significant and determine the best combination of input factors to achieve the desired quality of the final product
- Response Surface Analysis (RSM): This DoE was introduced by G.E. P Box and K.B. Wilson. RSM evaluates and runs a series of full factorial experiments and analyzes the response to generate mathematical equations that describe how factors affect response.
Software for DoE
Given below are some statistical software available in the market for DoE which have shown promise. It is difficult to conclude which software is the best and which one is not. The ultimate decision lies with the end-user.
- Minitab: Minitab helps to create, analyze and evaluate the designed experiment, which is similar to all types of designs, screening, factorial, response surface
- Design expert: Offers screening of factors up to 50. Evaluation of these factors on desired outcomes is analyzed using ANOVA
- JMP software: SAS created JMP in 1989 to help scientists and engineers visualize the data. Most of the advanced tools are available to create, analyze and optimize DoE problems
- MODDE: One advantage of MODDE for new users is that this software recommends a suitable design for the given set of parameters and levels. Sartorius builds the software and consists of all basic and advanced designs. It also supports CFR Part 11 audit trail.
Conclusion
DoE has received wide acceptance as the predominant tool for process optimization in the pharmaceutical industry. Chemical engineers generate the design models using screening and optimization based on data and knowledge (material attributes and process parameters) provided to them by chemists. The chemists would have extracted this data from basic and applied research, in order to solve real-world problems.
If these validated experimental models can be reproduced successfully, then the time required for developing insightful data can be reduced.2 As Aggarwal and Owen claimed, “The design models generated by both screening and optimization studies, are an approximation of reality. They are used to figure out the favoured settings, and then this setting can be implemented to validate the model”.3
In such a situation, it is recommended that the chemist and the chemical engineer ensure that both their process parameters are based on DoE. Their results should also be feasible, reproducible, and compatible with the project objectives. Only then can DoE ensure process development that is profitable in the long run.
To know about our Chemical Development services, click here
References
1. Khanam N, Alam M. I, Md Yusuf Ali, Q. M. A. I., & Siddiqui, A.- ur- Rahman. (2018) Review on Optimization of Drug Delivery System with Experimental Designs. International Journal of Applied Pharmaceutics, 10(2), 7-12. Doi.org/10.22159/ijap.2018v10i2.24482.
2. Steven A. Weissman & Neal G. Anderson. (2015) Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications, Org. Process Res. Dev. 19, 11, 1605–1633, Doi.org/10.1021/op500169m.
3. Patravale.V. B, Disouza J. I, & Rustomjee M. T. (2016) Design of Experiments: Basic Concepts and Its Application in Pharmaceutical Product Development, Pharmaceutical Product Development. DOI:10.1201/B19579-10.